Abstract
Microplastic particles (MPs, <5 mm) are found in marine ice in larger quantities than in seawater, however, the distribution pattern within the ice cores is not consistent. To get insights into the most general physical processes behind interactions of ice and plastic particles in cool natural environments, information from academic and applied research is integrated and verified against available field observations. Non-polar molecules of common-market plastics are hydrophobic, so MPs are weak ice nucleators, are repelled from water and ice, and concentrate within air bubbles and brine channels. A large difference in thermal properties of ice and plastics favours the concentration of MPs at the ice surface during freeze/thaw cycles. Under low environmental temperatures, falling in polar regions below the glass / brittle-ductile transition temperatures of the common-use plastics, they become brittle. This might partially explain the absence of floating macroplastics in polar waters. Freshwater freezes at a temperature well below that of its maximum density, so the water column is stably stratified, and MPs eventually concentrate at the ice surface and in air bubbles. In contrast, below growing sea ice, mechanisms of suspension freezing under conditions of (thermal plus haline) convection should permanently entangle MPs into ice. During further sea ice growth and aging, MPs are repelled from water and ice into air bubbles, brine channels, and to the upper/lower boundaries of the ice column. Sea ice permeability, especially while melting periods, can re-distribute sub-millimeter MPs through the brine channels, thus potentially introducing the variability of contamination with time. In accord with field observations, analysis reveals several competing factors that influence the distribution of MPs in sea ice. A thorough sampling of the upper ice surface, prevention of brine leakage while sampling and handling, considering the ice structure while segmenting the ice core—these steps may be advantageous for further understanding the pattern of plastic contamination in natural ice.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The variety of properties of synthetic polymers is so advantageous for diverse human needs that their production has grown exponentially over the last decades (Plastics Europe, Plastics—the facts (2019)). As a direct but undesired consequence, plastics accumulate in oceans: they compose nowadays the major part of marine litter (van Sebille et al 2012, Cozar et al 2014, Bergmann et al 2015), while microplastics (MPs, <5 mm) are reported in all the oceanic environments (Booth et al 2017, Eriksen et al 2014, Geyer et al 2017, among many others). While potential threats of the presence of plastic and MPs in the environment are becoming obvious, an understanding of their transport and accumulation pattern remains challenging: a wide diversity of original plastic material properties and lack of information on their modifications under natural conditions require further interdisciplinary consideration.
Seemingly pristine high-latitude regions appeared to be contaminated by plastics as well, and recent observations indicate that the MPs abundance in polar sea ice is orders of magnitude larger than that in surface waters (Obbard et al 2014, Kanhai et al 2020). Observations in icy environments are very difficult, laborious, and expensive, so only a few papers on field evidence of plastic contamination in sea ice have been published up to now (Obbard et al 2014, Peeken et al 2018, Geilfus et al 2019, Kanhai et al 2020, Kelly et al 2020, Kim et al 2021). Natural sea ice is by far more complicated than 'natural' plastics, which makes understanding MPs distribution in sea ice and its underlying physical background even more difficult. Dealing with a question of plastic/MPs contamination and its distribution in icy marine environments, one has to account for (i) the diversity of properties of plastics/MPs in the marine environment, (ii) the complexity of the sea ice structure and processes of its formation/modification/melting, and (iii) the information on field evidence, which is still rather limited. With sea ice formation and properties, there is already quite a long-lasting history of academic and industrial research, while for plastics a variety of applied investigations are available, like those exploring the material hydrophobicity or developing ice-preventing coatings or membranes. Integration of information from such diverse sources would be very useful for environmental applications. In this paper, an attempt is made to analyze some of the most common properties of plastics and ice, and some environmental processes that could be important for plastics/ice interactions under typical low-temperature oceanographic conditions. Obviously, at its first steps, such an analysis has to be limited to the consideration of just common properties, general principles, and typical conditions only.
Both plastics and sea ice are by themselves very complicated materials, with diverse physical, mechanical, thermal, and chemical properties. Recent peer-reviewed publications on properties of common-market plastics (using mainly industrial applications, 32 in total) and on marine ice (which address both academic and applied questions, 24 papers) were examined. Scopus database was used for the initial selection of the papers; their reference lists were employed for further clarification of the particular questions. Basic information from 7 textbooks was used, and simple illustrative laboratory tests were performed to highlight the most illuminating effects. It is shown that the hydrophobicity of common plastics, their brittleness under low temperature, and large thermal deviation from ice influence MPs fate in icy environments. Freeze-thaw cycles and processes of formation of ice in fresh and saline waters are of primary interest for MPs capture and distribution within the ice. To obtain most general conclusions, all the variability of possible properties of plastics, modifications of ice, specific environmental conditions, etc, is often disregarded, and the terms are preferentially used in their generic sense (unless otherwise stated): e.g., under 'plastics' are understood both synthetic and semi-synthetic polymers, 'polyethylene' denotes all the polyethylene family, etc The conclusions are then verified against the features of the contamination pattern observed in marine and lacustrine ice and polar waters (Obbard et al 2014, Waller et al 2017, Peeken et al 2018, Geilfus et al 2019, Hoffmann et al 2020, Kanhai et al 2020, Kelly et al 2020, Kim et al 2021, Wang et al 2021, Yakushev et al 2021).
2. Plastics: properties relevant to conditions in cool marine environments
In order to gather information on properties of plastics relevant to the particular questions of their interaction with natural ice, 67 peer-reviewed publications, and 7 books were used from different research fields, such as polymer chemistry, medicine, tribology, environmental protection, solar energy harvesting, etc None of them addressed problems related to physical oceanography directly, so simple laboratory visualizations were used to illustrate the relevant points.
2.1. Plastics hydrophobicity
High consumer qualities of common-market plastics are largely related to their low wettability (i.e., the hydrophobicity). Hydrophobic polymers encompass most widely used materials such as polyethylene, polystyrene, polyvinylchloride, some polyesters, poly(ethylene terephthalate), some polyurethanes, some polypropylenes, acrylics, nylons, and many others (Brash, 1979, Caron et al 2007, Law, 2014, Ariono and Wardani, 2017). By definition from chemistry and medicine, the hydrophobicity is the association of nonpolar groups or molecules in an aqueous environment which arises from the tendency of water to exclude nonpolar molecules (Caron et al 2007). Physically, there is no repulsive force involved in this process: it is an absence of attraction of nonpolar molecules of plastic material to polar molecules of water, while polar water molecules are attracted to one another. Hydrophobic molecules or groups (being excluded from water medium as much as possible) often cluster together.
Hydrophobic nature of the interaction of plastic and water molecules is of direct importance at the beginning of the ice formation - during the nucleation. As a general rule, the closer the structure of the crystal lattice of the seed to the lattice of ice, the greater the catalytic effect of such a seed. Natural waters contain a lot of effective organic and inorganic seeds/nucleators (of the size of ca. 10−4–10−3 cm) (Michel, 1978). Huge non-polar hydrophobic plastic molecules are much weaker nucleators than natural particles, and thus MPs in natural waters are supposed to be surrounded by ice crystals (i.e., frazil ice—needles, platelets, discoids), growing around other nucleators.
Hydrophobic surfaces have, by definition, little affinity with water (Law, 2014). Since plastic/MPs surfaces in the marine environment are permanently modified by weathering, mechanical impacts, or biofouling, their hydrophobicity can vary with time. Hydrophobicity is a very important characteristic of the surface, and it has attracted extensive attention from both fundamental and practical studies. When developed for particular applications, plastic surface wettability/hydrophobicity can be altered (e.g., (Wagner and Theato, 2014)), or surface from hydrophobic material can be converted to hydrophilic one using a specific coating (e.g., (Knaus and Nennadal, 1998, Jha et al 2016)). Leaving further details to more specific publications (e.g., (Caron et al 2007, Law, 2014), among many others), consider the influence of common-use polymers hydrophobicity on the behavior and fate of MPs particles in their interactions with natural water and ice.
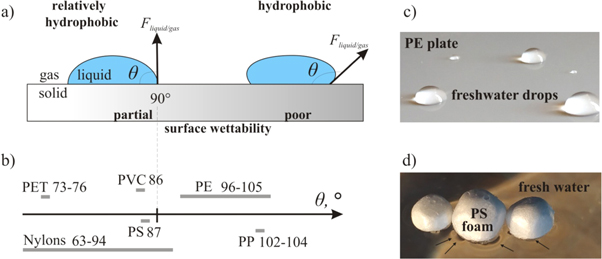
A quantitative parameter, characterizing surface hydrophobicity is the static water contact angle, θ (figure 1): the surface is called hydrophobic (poorly wettable) if θ is >90°, and relatively hydrophobic (partially wettable) if θ is around 90°. Examples of the contact angles measured for surfaces of materials made of some common-use polymers (Shadpour et al 2006) are shown in figure 1. For some polymers, quite a wide range of θ is shown: this is due to the fact that the contact angle characterizes the affinity of water with the particular surface, i.e., important are both the chemical composition of the material (with its additives) and the particular surface texture.
Figure 1. Hydrophobicity of plastics: (a) definition of the contact angle, θ, as the angle (within liquid phase) between two interfaces: solid-liquid and liquid-gas; (b) common plastics are characterized by θ more than or around 90°; (c) photo of fresh-water droplets on PE surface; (d) photo of PS-foam particles, which have substantially deformed water surface and (being repelled from water) tend to stick together.
Download figure:
Standard image High-resolution imageIt is known that increasing surface roughness amplifies natural surface chemistry (Jha et al 2016), so a rough hydrophobic surface has an even larger θ than a smooth one. In our case of MPs in the marine environment additives are known to leach from plastic matrices (e.g., (Andrady, 2011)), making the hydrophobicity of the very plastic material (with its non-polar molecules) of major importance. Moreover, the surface of MPs particles in the environment gets weathered and degraded, i.e., more rough (Corcoran, 2020), thus becoming even more hydrophobic (Jha et al 2016). When compared to freshwater, oceanic water has (slightly but) larger surface tension due to dissolved salts (Zhang and Carloni, 2012, Nayar et al 2014). Both these factors lead to a further decrease in particle wettability. On the other hand, the surface wettability of MPs in natural waters can be increased by attached biofilms and biofouling (Genzer and Efimenko, 2006).
Summing up, the hydrophobic nature of the interaction of non-polar plastic molecules with water remains a solid fact, while the exact value of the contact angle as the measure of the given plastic/MPs surface hydrophobicity provides only a proxy for environmental plastic particles. The contact angle can be a priori larger or smaller than the tabular value for the given plastic material due to the additives and the particular particle's surface texture (its roughness and homogeneity). It can vary also with time due to many reasons (e.g., leaching of additives, surface texture modification with weathering, attaching of biofilms).
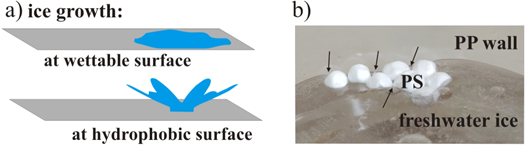
The influence of the substrate surface hydrophobicity/wettability on the beginning of the ice formation was studied by Liu with co-authors (Liu et al 2017) using laboratory and numerical experiments. They show that the surface wettability profoundly affects the orientation of the preferential growth face of ice crystals (Liu et al 2017): hydrophilic (wettable) surface dictates the along-surface growth mode (ASG), while at the hydrophobic surface the ice crystals show the off-surface growth (OSG) (figure 2(a)). Off-growing ice crystals have relatively little contact area with the surface, so even light wind (∼5 m s−1 in their experiments) easily removes them from the surface. Using experiments and simulations with surfaces of different roughness (pore size), Liu with co-authors (Liu et al 2017) showed that on the smooth surface the OSG mode occurs when the surface has a contact angle of more than 33°, with even lower contact angles for larger surface roughness. In other words, the so-called icephobicity (Hejazi et al 2013, Irajizad et al 2019) is observed already for values of θ definitely smaller than those indicated in figure 1(b) for common-use plastics, i.e., the off-surface growth of ice crystals is characteristic of plastic surfaces. The contact between an ice crystal and a plastic surface is relatively weak (Liu et al 2017).
Figure 2. Icephobicity of plastics: (a) Along-surface and off-surface growth of ice crystals (Liu et al 2017) at surfaces with θ below and above ∼33°, correspondingly; (b) curved ice surface around spherules of PS foam, which stick together and to the plastic (PP) walls of the container, but are loosely attached to the ice surface.
Download figure:
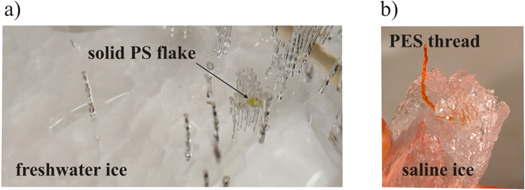
Standard image High-resolution imageThe direct consequence of plastics hydrophobicity and icefobocity is the tendency of growing ice to press MPs out—into air bubbles, in-between ice platelets, into brine channels. Figure 3 shows examples of laboratory visualization tests of this effect: in freshwater ice, the MPs particles (polystyrene, polyester) are exclusively associated with air bubbles, while in saline ice a polyester thread is (loosely) captured within the brine channel in-between ice platelets. Field substantiation of this effect is not yet available in scientific literature.
Figure 3. Coloured MPs particles visualize the effect of pressing them out of growing ice (a) into air bubbles of freshwater ice; (b) into the brine channels in-between ice platelets of saline ice.
Download figure:
Standard image High-resolution image2.2. Heat conductivity / heat capacity
Presence of solid contaminant particles near the ice surface influences absorption/reflection of solar radiation and can this way significantly change surface heat fluxes (Geilfus et al 2019). It has been shown already (Geilfus et al 2019) that presence of MPs in ice does not significantly influence its albedo and the growth rate even when MPs abundance is much larger than that observed in natural ice. Still, the difference in thermal properties between plastics and water/ice is important for the fate of MPs at the water/ice surface, and especially during freeze/thaw processes. Neither water/ice nor plastics are active heat sources in the considered case: heat is supplied to the surface ocean by external factors (solar radiation and exchange with the atmosphere), thus, a large difference in thermal response is expected to cause certain effects. For comparison, approximate values/ranges of heat capacity and heat conductivity of marine ice, ocean water, and common-use plastics are provided in table 1.
Table 1. Thermal properties of ice, water, and common-use plastics (indicative values).
| Material / Polymer family | Specific heat capacity at 0 °C, Cp J / (kg °C) | Heat conductivity, W / (°C m) |
|---|---|---|
| Sea ice (0–−15 °C; 2–20 psu) | 2010–2300 | 2.1–2.3 |
| Ocean water (0.01 °C, 34 psu) | 2050 | 0.56 |
| Snow | 2090 | 0.05–0.25 |
| PE | 600–1500 | 0.3–0.5 |
| PP | 300–2000 | 0.1–0.22- |
| PS | 900–2000 | 0.1–0.2; expanded 0.03 |
| Polyamides | 1600–1700 | 0.25 |
| Polyester | 900–1900 | 0.05 |
| PET | 1200–1350 | 0.15–0.4 |
Specific heat capacity of ice, water, and snow (without considering phase changes) is more than 2 kJ / (kg °C) (Hutter et al 2014), while for plastics it is substantially lower (roughly, below 1 kJ / (kg °C) (Brandrup et al 2004)). This means that, at the same external heat supply, the temperature of plastics rises (twice) faster than that of ice, snow, or water. Note, these are only estimates: (i) heat capacity of all substances depends on temperature, (ii) plastics have very diverse properties already at the production, and they are modified with time spent in the environment, and (iii) in our case, the phase changes in water/snow/ice system (when they take place) are dramatically important. Change of water phase (water/ice) requires huge amounts of energy (ca. 340 kJ / kg, supply or removal), which in both cases work to smooth out temperature variations. Namely, when water in, e.g., a lake begins freezing by cooling from the surface, the heat must first be removed to transfer water to ice—and only then temperature (of ice) begins decreasing. Similarly, when the ice melts, a lot of heat supply is required to first melt it—and only then (water) temperature begins rising. This tremendously high (compared to other substances) water phase-change barrier is known to be vital for marine life adaptation, while for plastics it does not take place (at ∼0 °C), which leads to a faster response of temperature of plastics to external heat fluxes compared to surrounding water or ice.
More than an order of magnitude difference in heat conductivity of plastics and water/ice (Brandrup et al 2004, Hutter et al 2014) also contributes to the formation of temperature differences between them. Heat conductivity of bulk polymers is usually very low, on the order of 0.1–0.5 W·m−1 C−1, which is generally the result of the complex morphology of polymer chains (Huang et al 2018). Heat conductivity of plastics depends on their crystallinity (and some other factors); that of the ice - on its porosity, salinity, and temperature. Heat transport in water is complicated by possible convection or turbulent mixing. However, the main rule is that the thermal signal propagates through water/ice much faster, while surface temperature of plastics is conducted into the bulk of the particle relatively slowly. Important for our consideration, the differences in both heat capacity and heat conductivity under naturally variable external heat fluxes favour the formation of temperature gradients between plastics and ice. (This effect is observed for many other floating particles: it has its roots in water/ice properties.)
The peculiarities of the thermal response of floating plastics and ice become especially evident in freeze/thaw cycles. Due to the hydrophobicity (and icephobicity) of plastics, the MPs particles are pushed out of ice when water freezes, but, due to faster thermal response, they become the 'centers of melting' when the heat is supplied. Photos in figure 4 illustrate the result: while warming (both from the air or the Sunlight), plastic/ice interface melts and plastic items become surrounded by water belts. In our tests, when freeze/thaw cycles were repeated, plastics were step-by-step pushed higher up from the level of the ice surface. As it was confirmed by observations in mesocosms (Geilfus et al 2019), surface MPs are very weakly attached to ice. Polystyrene foam bubbles, exposed to open-air conditions in our experiments, were removed from the ice surface by light wind after two nights (freeze/thaw cycles). In field, the particles at the surface may be covered in snow, and become part of the upper ice layer with further thaw/freeze event. This highlights that under the 'surface' of natural ice one should in fact understand a surface layer, directly influenced by the conditions at the ice/air interface.
Figure 4. Plastic items at the surface of melting freshwater ice: (a) PE lid melting out of ice at in-door conditions; note the significant distance between the ice and plastics (polyethylene lid and styrene-acrylonitrile wall of the container); (b) ice at the lake surface begins melting around fragments of floating litter.
Download figure:
Standard image High-resolution image2.3. Brittleness of plastics at low temperatures
Low temperatures and icy environments are challenging for most materials, and plastics are no exception. Decreasing temperature restricts molecule mobility and hence makes a polymer more brittle (Brostow et al 2011), which enhances the fragmentation of macro-plastic items and generation of MPs.
State of plastic brittleness is generally described in relation to specific temperatures (e.g., see (Brostow, 2001, Brandrup et al 2004)): the glass transition temperature, Tg, usually applied for polymers with generally amorphous structure (e.g., PS, PC, PVC, rubber) or by the temperature of the brittle/ductile transition, Tbd, used for semi-crystalline polymers (e.g., PP, HDPE, LDPE, PET). Physically, Tg and Tbd characterize different transitions in the state/behaviour of polymers: the glass transition temperature is a characteristic of polymer chains dynamics, whereas brittle/ductile transition temperature characterizes the polymeric material impact resistance (Brostow et al 2011). In typically structured polymers, crystalline domains, where polymer chains are aligned periodically, are surrounded by amorphous domains where the polymer chains are randomly entangled. Thus, any semi-crystalline polymer (e.g., polyolefins) has also its amorphous parts, so Tg can be applied (to its amorphous phase) along with Tbd. The brittleness of the polymer (along with other mechanical properties) is dependent on both its crystalline and amorphous phases (Sobjeraj, Rimnac, 2009). Seeking for crude indicative conclusions only, very simplified backgrounds are outlined here, sending the reader for details to specific literature on the glass and brittle/ductile transitions of polymers, e.g., (Brandrup et al 2004).
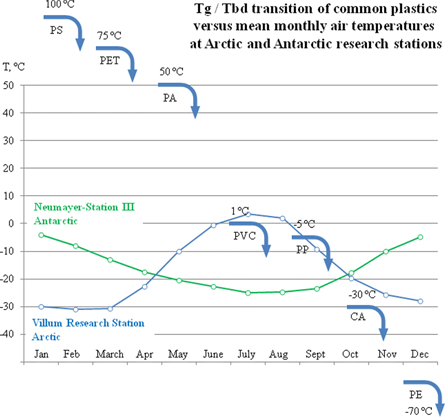
It is accepted that plastic materials at temperatures below their Tg/Tbd are brittle (Brostow et al 2011). Among amorphous polymers, some are made to be used in their 'glassy' state (i.e., below their Tg, like rigid PS), some are utilized in their rubbery state (i.e., above their Tg/Tbd, like polyisoprene, polyisobutylene, PP, most of PE). For semi-crystalline polymers, the temperature of transition between the brittle and ductile behaviour is usually not a specific value, but rather an interval of about 10 °C. Moreover, Tg/Tbd are physically dependent on numerous factors like polymer molecular weight, chain structure and morphology, additives used, degradation state, external contamination, strain rate, cyclic loading, triaxial tension, etc (e.g., (Wondraczek et al 2004, Sobieraj and Rimnac, 2009, Li et al 2021). The brittleness of plastics increases with aging (Brostow, 2001, Brostow et al 2011). It is also different for thin film (or coating) and balk materials because the film thickness and the nature of the substrate are linked with the crystal orientation and morphology (Forrest et al 1997). Some plastics are co-polymers, i.e., two (or more) different types of monomers are linked in the same polymer chain (McKeen, 2009), or polymer blends (Kalogeras, 2016), or there are multilayered or fiber-reinforced composites (Li et al 2021). It is known (e.g., (Brostow et al 2011, Kalogeras, 2016) that a change in proportion in co-polymers and blends can lead to a change in the balance of properties and hence influence the brittleness of the material. Practically, the brittleness of the material is defined by its weakest compound. With all these conditions in mind, the application of the exact values of Tg and Tbd to plastics in the environment is not reasonable. Some indicative values of Tg and Tbd for common-use polymers are shown in table 2. Taken for illustration, the comparison of these indicative values is shown in figure 5 with mean monthly air temperatures in the High Arctic (Greenland, 81° 36' N) at the Villum Research Station of Aarhus University, Denmark, and in the Antarctic (70° 41' S) at the Neumayer III Station of Alfred Wegener Institute, Germany (data from https://data.europeanpolarboard.org/). The majority of common-use polymers under such temperatures are in their brittle mode for a considerable part of the year. Synthetic fibers, due to the production technology, are usually highly crystalline, and their Tbd is typically well below naturally relevant temperature values.
Table 2. Some values of Tg (applicable to the amorphous part of polymer material) and Tbd (applicable to its crystalline part; highlighted in bold) for common-use polymers typically found in marine environment. Should be regarded as indicative rather than definitive.
| Polymer family | Tg, Tbd (approx.) °C |
|---|---|
| PS | +100 |
| PET | −40 ... +75 |
| Polyamides | −105 -- −65 ... +50 |
| PVC rigid | −10 -- +1 |
| PP | −20 -- −5 |
| Cellulose Acetate | −30 |
| PE | −70 |
Figure 5. Below the indicated temperatures plastics are mechanically brittle. Mean monthly air temperatures at research stations in the Arctic and Antarctic are shown for comparison (data from https://data.europeanpolarboard.org/).
Download figure:
Standard image High-resolution imageAlong with higher brittleness at low temperatures, freeze-thaw circles are shown to fasten the plastic material aging (e.g., (Kablov and Startsev, 2021)), while ice formation inside cracks favours mechanical fracture of the items. These effects should lead to much higher plastic fragmentation rates in polar oceans.
3. Natural ice formation, modification, melt: processes relevant to distribution and fate of MPs
Up to now, only a few papers have reported observational evidence of the presence of MPs in natural ice (Obbard et al 2014, Waller et al 2017, Peeken et al 2018, Geilfus et al 2019, Hoffmann et al 2020, Kanhai et al 2020, Kelly et al 2020, Kim et al 2021, Yakushev et al 2021)Wang et al 2021). In addition, three experimental works were carried out in mesocosms and laboratory, exploring the potential influence of MPs on ice albedo (Geilfus et al 2019, Hoffmann et al 2020), interactions between the ice algae and microplastics in sea ice (Geilfus et al 2019, Hoffmann et al 2020), and the fate of micro- and nanoplastics during sea-ice formation (Pradel et al 2021) With such a limited observational background, the analysis of the considered above plastic and ice properties might provide certain insights into the driving mechanisms behind the observed contamination pattern. Consider now the physical processes of the ice formation in fresh and saline waters, along with some aspects of MPs incorporation into the ice—and verify the conclusions against the available observational facts.
3.1. Vertical distribution of MPs in ice cores
Vertical distribution of MPs in sea ice cores was addressed in five investigations, all rejecting any consistent MPs distribution pattern. In Arctic Central Basin, (Peeken et al 2018) reported highly variable and 'unique MP patterns in different sea ice horizons' of pack and land-fast ice. And 'no consistent pattern' was noted by (Kanhai et al 2020). In the land-fast ice of the Gulf of Bothnia in the brackish Baltic Sea, (Geilfus et al 2019) found a more or less homogeneous abundance of MPs throughout the entire ice thickness and between stations. For the land-fast sea ice in East Antarctic, (Kelly et al 2020) stated that the sea ice does not have uniform MP concentrations throughout its thickness. In seasonal sea ice of the western Arctic Ocean, (Kim et al 2021) have not found drastic differences between top and bottom layers in any cores. It can thus be concluded that in the sea ice the MPs abundance has no certain fixed pattern, rather it depends on external conditions and the thermodynamic history of the particular ice (Geilfus et al 2019, Kanhai et al 2020).
The only work was published up to now reporting the distribution of MPs in freshwater ice cores: Wang with co-authors (Wang et al 2021) found a higher abundance of MPs in surface and bottom layers of the ice cover of shallow Lake Wuliangsuhai (China) than in the middle of the ice.
Seeking for possible explanations, consider now the process of the ice formation and potential behavior of MPs particles during this process. Entrapment of particles into ice takes place during its formation and growth (Michel, 1978, Obbard et al 2014, Peeken et al 2018, Bergmann et al 2019, Kanhai et al 2020). As it follows from the considerations above (section 2.1), MPs particles in natural environments can hardly serve as the centers of crystallization or substrates favourable for the ice formation. Plastics hydrophobicity and icefobicity determine also the tendency of ice, when it is formed, to press MPs particles out (as it happens to many other natural particles). Thus, the entrapment of MPs into ice takes place during further consolidation of the frazil platelets, which proceeds differently in fresh and saline waters due to (i) different mixing regimes of the underlying water column, and (ii) presence of brine rejection in saline waters. In contrast to solid freshwater ice, sea ice is permeable, favouring further re-distribution of particle-bearing brine through the network of channels.
3.2. Mixing regime of underlying water
Freshwater has its temperature of the density maximum (3.98 °C) well above the freezing point (0 °C), so the water column is thermally stably stratified while freezing from the top. In the absence of vertical convective mixing, particles suspended in the water column are naturally distributed in accordance with their buoyancy. That is, floating material concentrates near the surface, being entrained in vertical excursions below water surface only by possible wind-wave action.
Fresh-water ice commonly is a solid block, often transparent (remember wonderful photos of Lake Baikal's ice), which includes air bubbles (or, e.g., methane (Granin et al 2019)) and materials floating near the surface. Our laboratory tests confirmed two ways of entrapment of MPs particles into fresh-water ice: floating material (PE, 0.94 g/cm3) remained at the surface (loosely attached to the ice), while some of the sinking particles (PS, 1.05 g cm−3) captured within the ice were associated with air bubbles (see figure 3). In natural conditions, bubbles may originate from gases dissolved in water, or from biogeochemical processes in the water column or at the bottom.
For oceanic water (more precisely, for water with salinity more than 24.6), the temperature of the density maximum is below the freezing temperature (Hutter et al 2014), so ocean water experiences thermally-induced vertical convection while cooling down to the freezing point. The reported vertical velocity in convective plumes in polar seas (without the ice formation) under surface cooling conditions may reach up to 10 cm s−1 (Marshall and Schott, 1999). With the beginning of the ice formation, thermally-induced convection in the water column somewhat decreases, since ice restricts heat exchange between water and the atmosphere. However, brine rejection leads to an increase in destabilizing buoyancy flux, and vertical velocities may also reach several units of cm/s (McPhee and Stanton, 1996).
Several studies reported that the prevalent MPs type in polar waters is fibers, while the size of fragments is below 2 mm (Peeken et al 2018, Kelly et al 2020, Suaria et al 2020). These facts can be used to evaluate the potential sinking/rising velocities of typical MPs in polar regions. The characteristic sinking velocity of thin synthetic fibers is shown to be about 1.6 mm s−1 (Khatmullina and Chubarenko, 2021), while for most heavy (PET, PVC) spherules of the size less than 2 mm the sinking velocity can be up to 10 cm s−1 (Chubarenko et al 2016, Khatmullina and Isachenko, 2017). Thus, MPs particles, with their typical sinking/rising velocities of units of mm/s, should be kept suspended in the water column when the ice is growing. Investigations of the mechanism of sediment incorporation into sea ice, referred to as suspension freezing (e.g., (Dethleff et al 2009, Ito et al 2019), among many others), show that underwater interaction between frazil ice and suspended particles is an efficient mechanism of particle entrapment into the ice. Under laboratory conditions, the number of (heavy and relatively rounded) sediment particles entrained by convective mixing into newly formed ice was more than twice larger than the suspended particle concentration in water (Dethleff et al 2009). Physically, this happens because under (inherently unsteady) convective mixing particles with different properties trace different orbits, i.e., are effectively intermixed (Dethleff et al 2009). For plastic micro- and nanoparticles in the environment, natural organic matter (Pradel et al 2021) and interactions with algae (Hoffmann et al 2020) play a significant role in the uptake of particles into the sea ice. These factors may in part explain why MPs concentrations in ice are larger than those in water: Kanhai with co-authors (Kanhai et al 2020) reported that MPs abundance in Arctic surface waters underlying ice floes (0–18 particles m−3) was three orders of magnitude lower than that in sea ice cores (2–17 particles L−1), while (Kelly et al 2020) came to a similar conclusion for the Southern Ocean. It was also shown (Kanhai et al 2018) that the Polar Mixed Layer (the upper several tens of meters) has the largest MPs concentrations (up to 375 particles m−3), while below it the MPs abundance did not exceed 104 particles m−3, which most probably is due to retention of MPs by vertical convection (Khatmullina and Chubarenko, 2021). Similarly, for the freshwater environment, (Wang et al 2021) found that ice was approximately 10–100 times more contaminated with MPs than waters below it. Vertical distribution of MPs in sea ice formed from brackish water (the salinity range between 0 and 24.6) was examined in the Baltic Sea by Geilfus with co-authors (Geilfus et al 2019). For such waters, the situation is transitory. Surface water freezing begins at (weak but) stable thermal stratification (similar to lakes), but with the initiation of the ice formation, the rejection of brine easily overcomes the stabilizing effect of thermal stratification, since the contribution of temperature to the variations of water density is known to be small compared to the influence of water salinity in the Baltic Sea (Chubarenko and Demchenko, 2010, Chubarenko and Stepanova, 2018). Taking into account severe wind/wave conditions at mid-latitudes, vertical mixing of the water column can be assumed as the background process for the incorporation of MPs into ice both before and after the beginning of freezing. This way, the distribution of MPs in the ice cores of the Baltic Sea (Geilfus et al 2019) should be indeed more similar to the oceanic than to the lacustrine case.
3.3. Sea ice structure and permeability
In contrast to solid freshwater ice, sea ice is a complex multiphase medium, containing crystals of practically fresh ice, a liquid phase, salts in a dissolved and solid state (Worster, 1997, Feltham et al 2006, Worster and Rees Jones, 2015, Wells et al 2019), and various impurities like air bubbles, small algae, mineral solids, and now MPs particles. Investigations of sea-ice growth and melt have received the highest attention recently (e.g., (Krembs et al 2000, Lieblappen et al 2018, Rees Jones and Wells, 2018, Wells et al 2019) among others), addressing also the questions of ice porosity, permeability, and convective flows of gas bubbles and brine through porous ice. All these processes are capable of redistributing MPs particles within the ice, especially during the ice melt and freeze/thaw cycles, so they are briefly touched below. Since the orientation and time rate of growth of ice crystals are very sensitive to external conditions (such as heat fluxes, mechanical loads, particular salts phase changes, the presence and chemistry of impurities, underlying water stratification, etc), the vertical structure of the ice column is always complex. Essentially, sea ice is a mushy layer (Feltham et al 2006) comprising a porous matrix of ice crystals filled with brine, concentrated in interconnected brine channels and pockets. Their shapes and sizes are very diverse, from flat interlayers or filamentary inclusions between crystals with a cross-section of several microns to semi-spherical pockets and large top-to-bottom channels filled with brine with a volume of several cubic centimeters (Lieblappen et al 2018). The diameter of brine channels is normally less than 1 mm (Krembs et al 2000), but varying growth rates in natural sea ice lead to different spacing between brine layers and variable percolation with depth (Lieblappen et al 2018). In particular, a strong structural difference of brine channels networks between frazil and columnar ice in first-year ice samples is pointed out by (Lieblappen et al 2018): in vertical, brine pockets in frazil ice larger than about 1 mm tend to join or split while the largest brine pockets in columnar ice tend to persist and brine channels in columnar ice stretch end-to-end with little change in size. In response to warming, the number of interconnected brine pockets and channels increases (Light et al 2015), so that ice permeability also significantly increases (Widell et al 2006, Jardon et al 2013, Wells et al 2019).
Due to plastics' hydrophobicity, the MPs particles are pushed out of ice to brine channels, and the similar size ranges of MPs and brine channels make the migration of air bubbles and brine through the ice of potential importance to the vertical distribution of MPs within the ice. Experimental data show (Widell et al 2006, Jardon et al 2013, Wells et al 2019) that the brine flows out of polar ice not only during the period of its formation but also in summer: at high temperatures, ice bridges across brine channels melt down making ice much more permeable. This suggests possible seasonal variations of the MPs abundance. Thus, several reasons suggest that the distribution of MPs particles can vary in space and time: (i) variability of external conditions during the ice formation (e.g., buoyancy fluxes in the underlying water column, freeze/thaw cycles, etc), (ii) complex vertical structure of ice (frazil versus columnar ice; water / snow / ice over-topping due to motion of floes or weather changes, etc), and (iii) temporal (multi-year, seasonal, diurnal) variability of MPs content due to brine/air migration processes within the ice. With so many sources of variability, it is quite expectable that the first observations of the MPs distribution within sea ice (Obbard et al 2014, Peeken et al 2018, Geilfus et al 2019, Kanhai et al 2020, Kelly et al 2020, Kim et al 2021) did not encounter any distinct pattern.
Still, there are some indications that there could be more MPs particles closer to air/ice and ice/water interfaces, as it is observed for, e.g., oil and biota (Nemirovskaya et al 2015). Firstly, some plastics are positively buoyant and float at the surface, and there is a mechanism, pushing them further up during freeze/thaw cycles. Secondly, MPs are expelled from growing ice into brine channels, and brine is with time pressed out of ice both upwards and downwards (Jardon et al 2013). This, in particular, leads to the well-known C-shape of the salinity profile within the ice (Geilfus et al 2019). Small micro- and nano-plastics can thus be expected to be more abundant in the layers with higher ice salinity, while larger MPs (say, > 1 mm, taken here as an order of magnitude for the brine channel diameter) are expected to be captured within the columnar ice. Interestingly, laboratory experiments by Pradel with co-authors (Pradel et al 2021) have confirmed that microplastics (< 5 mm) are retained in saline ice while nanoplastics (<1 μm) are expelled from it. Analysis of MPs size distribution in ice cores from the western Arctic Ocean (Kim et al 2021) also showed that small particles below specific size classes might be more deficient than those observed in Eurasian samples or those expected from a fragmentation model. Fibers, since they are long and flexible, can be effectively captured within the brine channels network.
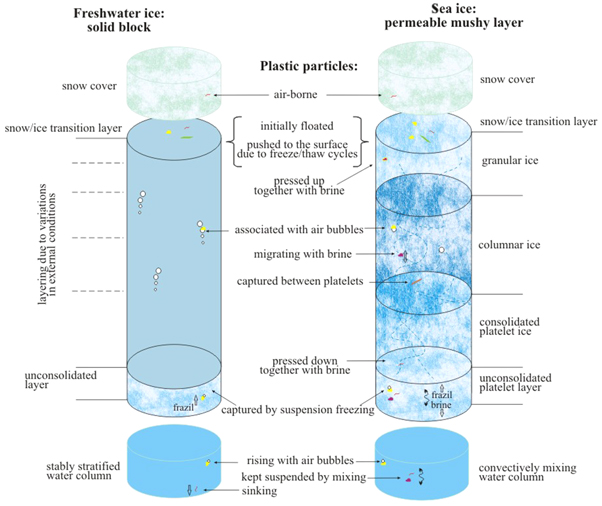
While for freshwater ice higher concentrations of MPs in upper and lower ice layers were already confirmed by field data (Wang et a., 2021), with sea ice the contamination pattern is more complicated. Field observations in oceans and mesocosm experiments provide some examples, which can probably be considered as underpinning the tendency for more MPs in the upper and lower ice layers. Among 25 ice cores analyzed by (Kanhai et al 2020), one core showed a significant negative correlation between MPs concentration and sub-section depth. Kelly with co-authors segmented the analyzed core of land-fast ice accounting for its structure (top 0 to 10 cm of granular ice, followed by 10–105 cm of the middle section with the columnar ice, and 105–115 cm bottom section, most biologically dense)—and got larger MPs abundance in the top and bottom sections (Kelly et al 2020). Geilfus with co-authors mentioned that in their mesocosm experiments there was a lot of plastics on the top of each core. The particles were loosely attached to the ice surface, were removed, and not accounted for in the further analysis of vertical MPs distribution within the ice (Geilfus et al 2019). Similar surface scrapping from snow and (possibly) air-borne particles was applied by (Kelly et al 2020). For future investigations on whether upper/lower ice layers are more contaminated by plastics, one should (i) thoroughly collect the material (snow&ice) from the top, (ii) to prevent as much as possible the leakage of brine from the core while sampling, and (iii) cut the core possibly in accordance with its structure. Summarizing the discussion, a simplified scheme of the vertical structure of freshwater and sea ice is provided in figure 6, together with indications of potential mechanisms of MPs entrapment and re-distribution.
Figure 6. A simplified scheme of the vertical structure of freshwater and sea ice. In the middle: potential mechanisms of MPs entrapment and re-distribution, revealed by the analysis in this study.
Download figure:
Standard image High-resolution image3.4. Absence of macro-plastics in polar waters: efficient fragmentation?
Several studies report little or no floating plastic macro-litter in polar waters of both hemispheres. For the Arctic waters, Cózar with co-authors pointed out that plastic debris (>0.5 mm) was scarce or absent, and only quite aged debris was abundant in the northernmost and easternmost areas of Greenland and the Barents Seas (Cózar et al 2017). Pogojeva with co-authors also confirmed that floating macrolitter (>2.5 cm) was found in their expedition (September–November 2020) only in the Atlantic water of the Barents Sea and the Kara Sea, while all the other parts of the Russian Arctic Seas east of the Gulf of Ob were free from floating litter (Pogojeva et al 2021).
Around Antarctica, very low concentrations of floating macroplastics were found by (Suaria et al 2020). Waller with co-authors concluded that macroplastics there is unlikely to originate from local sources due to low human activity so transport of macrolitter from other regions is expected to contribute to the contamination (Waller et al 2017). In contrast, Kelly with co-authors suggested local MPs contamination sources in Antarctica, since slightly larger plastic particles (compared to those found in Arctic ice (Peeken et al 2018)) were detected in the Antarctic coastal land-fast sea ice (Kelly et al 2020).
Among additional possible reasons for low littering of these remote regions, no input of large litter from Siberian rivers (at least in autumn) was mentioned by (Pogojeva et al 2021), while (Suaria et al 2020) pointed at the dynamic limitations put on floating items by Antarctic circumpolar fronts. Still, macroplastics is brought to the Arctic Ocean at least from the Atlantic region (Cózar et al 2017, Pogojeva et al 2021), and Suaria with co-authors (Suaria et al 2020) discussed storm-driven surface waves and ocean eddies as a possible mechanism facilitating the crossing of the polar fronts by floating debris. It looks like macroplastics are supplied to high-latitude oceans from temperate regions, but cannot survive there.
Comparison of mean air temperatures in polar regions with temperatures of the glass transition and brittle-ductile transition of common polymers (see section 2.3) suggests one possible explanation: plastics in low-temperature regions become mechanically brittle. Heavy mechanical forcing can be caused by waves at the coasts and by interactions with ice while floe movements or ice deformations. Laboratory experiments (Chubarenko et al 2020) showed that clastic shores (typical of high latitudes) are very effective in the fragmentation of macroplastics, and this is in accord with field observations (Convey et al 2002, Thiel et al 2013). Last but not least is high wave energy in polar oceans as a factor leading to enhanced plastic fragmentation along the coasts. If slightly rolling pebbles under moderate-energy laboratory conditions could fragment at room temperature 20, 30, up to 99.8% of plastics items (PP, LDPE, PS, correspondingly) into MPs during 24 h (Chubarenko et al 2020), at low temperatures and under high oceanic waves the time scales of fragmentation should be even smaller.
Mechanical impacts on plastics due to ice formation and motion should further fasten their fragmentation. In the case of the Arctic Ocean, plastic litter is expected to be supplied by large Siberian rivers, e.g., Ob, Yenisey, Lena, whose catchment harbors more than 38 million people. These rivers have their main discharge (40%–50%) in June due to spring thawing (Frolova et al 2021). More than half a year these rivers are ice-covered and have minimum water discharge, so plastic litter, potentially trapped within the river course, enters the Arctic Ocean together with moving ice floes. Yakushev with co-authors (Yakushev et al 2021) analyzed MPs characteristics in different water masses of the Eurasian Arctic and found a remarkable feature: the inner part of water plumes adjacent to the river estuaries and deltas had smaller-sized MPs (with particle surface area < 3 mm2) while the outer-plume MPs were distinctly larger (surface area up to 10 mm2). The overwhelming prevalence of polyester and polyamide fibers among MPs in cool polar waters (up to 93% in the Arctic Central Basin (Kanhai et al 2020)) might suggest enhanced fragmentation of other plastics to even smaller nanoparticles, while such (highly crystalline) fibers are still in their ductile mode and are thus more resistant to mechanical fragmentation in low-temperature environments.
4. Conclusions
Marine ice is suggested to be an important link in the chain of transport and accumulation of plastic particles in the ocean. Results of field investigations of ice contamination by plastics are alarming, but rather limited at present due to high costs and hard-to-reach locations. A preliminary analysis of physical processes behind interactions of ice and plastic particles provides a deeper understanding of the contamination pattern and reveals areas where further targeted research is required. Table 3 provides an overview of the discussed questions and lists some of the gaps in our present-day knowledge on the distribution of MPs in natural ice.
Table 3. Discussed questions and present-day knowledge gaps on properties/effects relevant to MPs distribution in natural ice cores. References to corresponding literature can be found in the sections, indicated in the first column.
| Section | Question | What is known | Knowledge gaps |
|---|---|---|---|
| 2.1 | To which extent is natural MPs hydrophobic/wettable ? | Plastic materials are hydrophobic | Influence of biofilms and organic matter |
| 2.1 | Are natural MPs repelled from ice to air bubbles and brine channels? | Yes for new MPs in laboratory | Field evidence required |
| 2.2 | Influence of MPs in ice on heat-flux-related phenomena | No influence on ice albedo and thawing due to small MPs concentrations in natural ice | Possible enrichment of surface layers by MPs during freeze/thaw cycles |
| 2.3, 3.2 | Aging/brittleness of plastic materials in icy environments | Applied case studies | Field evidence. |
| Possible specific behavior of co-polymers, polymer blends, composites. | |||
| 3.1 | Vertical distribution of MPs in ice cores | No consistent pattern in sea ice cores. | Higher MPs concentrations in surface/bottom ice layers and at structural interfaces are expected but not confirmed yet. |
| 3.1 | What is the influence of sea ice permeability? | Theoretical suggestion of size-specific fate of micro- and nano-plastics. | Potential temporal variation of MPs contamination level and its vertical distribution with ice aging. |
| Laboratory confirmation of the effect. |
For future field works, certain suggestions can be formulated for more effective sampling and handling schemes. Since vertical structure of natural ice follows changes in external environmental conditions, it is quite reasonable to analyze MPs contamination in accordance with structural layers (i.e., with variable step), with particular interest to interfaces between them. Thorough collection and analysis of the material (snow&ice) from the top is highly recommended. While sampling, it is important to prevent as much as possible the leakage of brine from the core. Sampling of additional ice cores is recommended to reveal (i) the salinity profile and (ii) the geometry of brine channels. It is reasonable to analyse MPs contamination of sea ice in at least two size fractions: larger particles captured within ice and smaller particles migrating with brine, with cut-off size prescribed by the diameter of brine channels.
Given that both natural ice and plastics have rather specific and diverse properties, variable with time, and influenced by external environmental conditions, only very general conclusions and only the main trends were formulated at the first steps. One more complication of such analysis is related to the fact that plastics properties are designed to suit external conditions which are far from the natural ones. Thus, neither manufacturers nor end-users have systematically examined behaviour and properties of common-use plastics in natural icy environments. For the particular case of interaction of plastic particles with ice, the required pieces of information were collected from both academic and applied investigations with the hope to support further environmental research and physical understanding.
Acknowledgments
Investigations are supported by the Russian Science Foundation, grant No 19-17-00041. Laboratory facilities are maintained under the State Assignment of ABIORAS No 0128-2021-0012.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).